When you buy through our links, we may earn a commission. Products or services may be offered by an affiliated entity. Learn more.
Sleep Apnea
- Overview: Sleep apnea is a sleep disorder that involves repeated lapses in breathing during sleep. The two main types are obstructive sleep apnea (OSA) and central sleep apnea (CSA).
- Symptoms: The most common symptoms are loud snoring, episodes of gasping or choking, and excessive daytime sleepiness.
- Causes: OSA is caused by the relaxation of throat muscles that obstruct the airway, while CSA is caused by an underlying medical issue that affects the brain’s respiratory control centers.
- Diagnosis: Diagnosis requires a sleep study, either in-lab polysomnography or a home sleep test, with results interpreted by a healthcare professional.
- Treatment: Continuous positive airway pressure (CPAP) therapy uses pressurized air to keep the airway open. Other options include oral appliances, lifestyle changes, and surgery.
While often dismissed as mere snoring, sleep apnea is a serious condition that affects an estimated 30 million adults in the United States. Sleep apnea causes frequent pauses in breathing during sleep. These breathing interruptions reduce the quality of sleep and, if left untreated, can lead to potentially serious health consequences.
If you think you may be at risk for sleep apnea, it’s critical to work with a doctor so that you can get any necessary testing and treatment. Below, we’ll cover what sleep apnea is, what the symptoms and causes are, and how it’s treated.
Don’t Just Test Your Sleep. Understand It.
Discover if sleep apnea is holding you back. You’ll get a personalized medical report and an expert consultation to help you take action.

What Is Sleep Apnea?
Sleep apnea is a type of sleep-related breathing disorder, a group of sleep disorders characterized by abnormal breathing patterns during sleep.
People with sleep apnea repeatedly have reductions or pauses in breathing for brief periods while they sleep. Although these lapses cause a person to awaken periodically and reduce sleep quality, sleepers may not fully wake up and remain unaware that their nighttime breathing is abnormal.
There are several types of sleep apnea, categorized by the cause of breathing disruptions.

Types of Sleep Apnea

Related Sleep Disorders
Sleep Apnea Symptoms
The symptoms of sleep apnea include the effects of abnormal nighttime breathing, as well as the daytime consequences of severely reduced sleep quality. Identifying these signs is crucial, as they’re often mistaken for simple tiredness or chronic snoring.
- Loud, chronic snoring
- Dry mouth or sore throat upon waking
- Excessive daytime sleepiness
- Gasping, choking, or snorting during sleep
- Morning headaches
- Difficulty concentrating or memory problems
- Irritability or mood changes
What Causes Sleep Apnea?
The underlying causes of sleep apnea are complex and stem from different physical or neurological issues that prevent the body from sustaining normal, continuous breathing while asleep.
Obstructive Sleep Apnea
In people with obstructive sleep apnea, the muscles in the back of the throat relax during sleep, reducing space for air to pass through. Snoring occurs as the airway narrows, and when the airway is obstructed, a person fails to get enough oxygen. The lack of oxygen causes partial or complete awakenings in order to restore airflow. These breathing disruptions happen repeatedly during sleep.
Central Sleep Apnea
Central sleep apnea arises because of problems in how the brain communicates with the muscles responsible for breathing. For people with CSA, a part of the brain called the brain stem fails to properly recognize carbon dioxide levels in the body during sleep. This leads to repeated episodes of breathing that’s slower and shallower than it should be.
Sleep Apnea Risk Factors
The likelihood of developing sleep apnea is influenced by a combination of anatomical, lifestyle, and medical factors that vary significantly between OSA and CSA.
Obstructive Sleep Apnea
The primary risk factors for obstructive sleep apnea are related to age, sex, body weight, and certain anatomical features of the head and neck area.
- Age: The risk of developing obstructive sleep apnea increases with age until a person is in their 60s and 70s.
- Sex: Men or people assigned male at birth are generally more likely to have obstructive sleep apnea, especially in the earlier stages of adulthood.
- Head and neck anatomy: Obstructive sleep apnea occurs more frequently in people who have specific anatomical features including a larger tongue and a shorter lower jaw.
- Body weight: Multiple studies have found a correlation between a higher body mass index (BMI) and an elevated risk of developing obstructive sleep apnea.
Central Sleep Apnea
Central sleep apnea most often occurs as a consequence of another medical problem, such as an infection or injury affecting the brain stem, heart or kidney failure, stroke, or excess growth hormone production. Studies have identified some additional factors that are linked with a heightened risk of central sleep apnea.
- Age: People who are over age 65 have a heightened risk of breathing disruptions consistent with central sleep apnea.
- Sex: Central sleep apnea is more common in men or people assigned male at birth, which may be related to levels of certain sex hormones.
- Use of certain drugs: Chronic use of opioid drugs and some prescription medications can affect breathing and have been associated with a higher risk of CSA.
- Being at high altitude: Spending time at high-altitude is associated with CSA because of the decreased availability of oxygen in high-altitude environments.
How Is Sleep Apnea Diagnosed?
Sleep apnea must be diagnosed by a doctor or sleep specialist, and there are several steps in the diagnostic process.
Doctor’s Visit and Symptom Review
The process begins with a detailed medical history and physical exam. Your doctor will assess your risk factors and review your symptoms, relying heavily on information provided by a bed partner, who can report signs like loud snoring or gasping.
Sleep Study
A sleep study is necessary to diagnose obstructive or central sleep apnea. The most dependable kind of sleep study is called polysomnography, which is conducted during an overnight stay at a specialized sleep laboratory. But there’s also the option to do a sleep study at home.
- In-lab sleep study: Multiple sensors are used to track breathing, awakenings, oxygen levels, muscle movement, sleep stages, and other aspects of sleep. An in-clinic sleep study can determine if breathing is abnormal and differentiate between obstructive and central sleep apnea. For OSA, polysomnography may involve either one or two visits to a sleep clinic.
- At-home sleep test: This is an option for certain patients who are believed to have more severe obstructive sleep apnea. Taking an at-home sleep test may be more convenient, but the results must still be interpreted by a health professional. Home testing isn’t used for central sleep apnea.
Results Interpretation and Diagnosis
After the sleep study, a sleep specialist analyzes the results. The key finding is the apnea-hypopnea index (AHI), which is the total number of apneas (pauses in breathing) and hypopneas (shallow breaths) that occur per hour of sleep. The AHI determines the severity of the diagnosis:
- Mild: 5 to 15 events per hour
- Moderate: 15 to 30 events per hour
- Severe: more than 30 events per hour
Sleep Apnea Treatments
The goal of treatment for sleep apnea is to reduce breathing disruptions and improve sleep. The approach to treatment varies between obstructive sleep apnea and central sleep apnea, but in all cases, effective management is crucial to prevent serious long-term health complications.

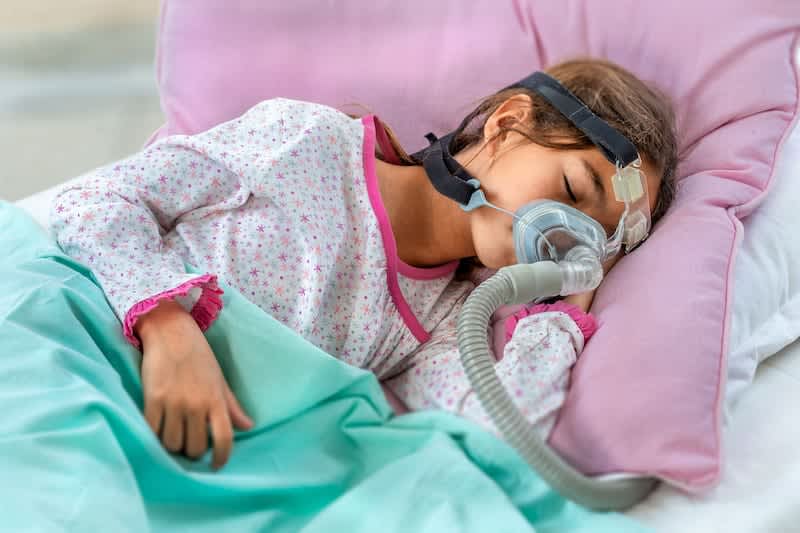
PAP Therapy for Sleep Apnea
Positive airway pressure (PAP) therapy is the most common and effective treatment for obstructive sleep apnea (OSA). A machine gently delivers pressurized air through a mask worn during sleep, keeping the airway open and preventing the breathing pauses characteristic of the disorder. There are variations like CPAP (continuous), BiPAP (bilevel), and APAP (automatic) depending on a patient’s specific needs.
Surgical options are generally reserved for patients who cannot tolerate PAP therapy or have specific anatomical abnormalities contributing to their OSA. Procedures can range from removing excess tissue in the throat (UPPP) to more advanced methods like hypoglossal nerve stimulation, which uses an implanted device to move the tongue forward during sleep.
Alternative treatment options for sleep apnea include oral appliances, which are custom-fitted mouthpieces worn at night to reposition the jaw and tongue to keep the airway clear, as well as lifestyle changes, such as weight loss, minimizing alcohol intake, and using positional therapy devices to avoid sleeping on the back.
Complications of Sleep Apnea
Effective treatment can generally prevent or resolve serious complications from sleep apnea, but if the condition is left untreated, it can have far-reaching effects on health and well-being. Accordingly, obstructive sleep apnea has been associated with a higher risk of a diverse range of health problems, including:
- Car accidents from drowsy driving
- Cardiovascular diseases like high blood pressure, stroke, heart failure, heart disease, and an abnormal heartbeat
- Metabolic disorders including type 2 diabetes
- Pulmonary hypertension, which is high blood pressure in the arteries of the lungs that places excess strain on the heart
- Thinking problems such as impaired memory and concentration
- Mood disturbances including irritability and a higher risk of depression
- Nonalcoholic fatty liver disease, which is an increase in fat deposits in the liver that can contribute to serious liver damage
- Anesthesia-related complications during surgery
Sleep Apnea in Children
Although frequently associated with older adults, sleep apnea can occur in children. In young people, obstructive sleep apnea is much more common than central sleep apnea. It’s estimated that 1% to 5% of children have obstructive sleep apnea.
Living With Sleep Apnea
Practical steps can help people living with sleep apnea to cope with this condition and its potential health effects.
- Work closely with a doctor: It’s important to stay in touch with a primary care doctor or sleep specialist and report any ongoing symptoms, challenges with treatment, or other concerns that may require an adjustment to the plan for managing sleep apnea.
- Properly care for treatment devices: Whether using a PAP device or a mouthpiece, cleaning and maintenance can help get the most out of treatment and avoid unwanted side effects.
- Avoid high-risk activities: People with sleep apnea should be aware of the risks of daytime sleepiness. Especially for people with untreated sleep apnea, activities like driving or operating heavy machinery should be avoided when drowsy.
- Consider changing sleeping position: Although they haven’t been rigorously studied, special products to avoid back sleeping may help some people reduce their symptoms from obstructive sleep apnea.
- Minimize alcohol consumption: Reducing alcohol intake can be a component of the treatment plan for sleep apnea. In people with untreated OSA, even daytime alcohol consumption may exacerbate breathing problems at night.
- Inform new doctors about sleep apnea: People with sleep apnea should mention this condition to any new medical providers, especially when planning to start a new medication or undergo surgery.
Frequently Asked Questions
Does snoring mean you have sleep apnea?
Snoring is the most common symptom of obstructive sleep apnea, but not everyone who snores has sleep apnea. Snoring occurs when air flow is partially obstructed, causing vibration. Only when the snoring is interrupted by episodes of gasping, choking, or pauses in breathing does it signal that you may have sleep apnea and require medical evaluation.
Is sleep apnea genetic?
Sleep apnea itself isn’t a single genetic disease, but the risk of developing it can be inherited. This is because many risk factors for obstructive sleep apnea are genetic, such as the natural structure of your jaw, neck, and throat anatomy. If close family members have sleep apnea, your risk is significantly increased.
Can sleep apnea kill you?
While a person isn’t likely to die suddenly from a single apnea event, untreated severe sleep apnea can significantly shorten your lifespan due to serious health complications. It greatly increases the risk of conditions like high blood pressure, stroke, heart attack, heart failure, and life-threatening accidents caused by drowsy driving.
Can sleep apnea be cured?
Sleep apnea is generally considered a chronic condition that must be managed, but it can be cured in specific circumstances. For obstructive sleep apnea, symptoms may be eliminated through surgical intervention (for those with specific anatomical issues) or significant weight loss. For most people, however, the condition is successfully managed using treatments like CPAP therapy.

Still have questions? Ask our community!
Join our Sleep Care Community — a trusted hub of sleep health professionals, product specialists, and people just like you. Whether you need expert sleep advice for your insomnia or you’re searching for the perfect mattress, we’ve got you covered. Get personalized guidance from the experts who know sleep best.
References
14 Sources
-
Frost & Sullivan. (2016). Hidden health crisis costing America billions: Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. American Academy of Sleep Medicine.
https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf -
National Institutes of Health (NIH) National Heart, Lung, and Blood Institute. (2022, March 24). Sleep apnea: What is sleep apnea? National Institutes of Health (NIH) National Heart, Lung, and Blood Institute.
https://www.nhlbi.nih.gov/health/sleep-apnea -
Malhotra, A., Kundel, V. (2024, December 17). Obstructive sleep apnea overview of management in adults. UpToDate.
https://www.uptodate.com/contents/obstructive-sleep-apnea-overview-of-management-in-adults -
Badr, M. S. (2023, March 9). Central sleep apnea: Pathogenesis. In R. D. Chervin (Ed.). UpToDate.
https://www.uptodate.com/contents/central-sleep-apnea-pathogenesis -
Dudley, K. A., & Patel, S. R. (2016). Disparities and genetic risk factors in obstructive sleep apnea. Sleep Medicine, 18, 96–102.
https://pubmed.ncbi.nlm.nih.gov/26428843/ -
A.D.A.M. Medical Encyclopedia. (2021, July 12). Central sleep apnea. MedlinePlus.
https://medlineplus.gov/ency/article/003997.htm -
Badr, M. S. (2021, September 30). Central sleep apnea: Risk factors, clinical presentation, and diagnosis. In R. D. Chervin (Ed.). UpToDate.
https://www.uptodate.com/contents/central-sleep-apnea-risk-factors-clinical-presentation-and-diagnosis -
Gallagher, S. A., Hackett, P., Rosen, J. M. (2022, February 11). High altitude illness: Physiology, risk factors, and general prevention. In D. F. Danzl (Ed.). UpToDate.
https://www.uptodate.com/contents/high-altitude-illness-physiology-risk-factors-and-general-prevention -
Borsini, E., Noguiera, F., & Nigro, C. (2018). Apnea-hypopnea index in sleep studies and the risk of over-simplification. Sleep Science, 11(1), 45–48.
https://pubmed.ncbi.nlm.nih.gov/29796201/ -
Mehra, R. (2021, August 25). Obstructive sleep apnea and cardiovascular disease in adults. In N. Collop (Ed.). UpToDate.
https://www.uptodate.com/contents/obstructive-sleep-apnea-and-cardiovascular-disease-in-adults -
A.D.A.M. Medical Encyclopedia. (2020, January 1). Pulmonary hypertension. MedlinePlus.
https://medlineplus.gov/ency/article/000112.htm -
A.D.A.M. Medical Encyclopedia. (2021, April 22). Nonalcoholic fatty liver disease. MedlinePlus.
https://medlineplus.gov/ency/article/007657.htm -
Constantin, E., Brouillette, R. T. (2022, February 18). Congenital central hypoventilation syndrome and other causes of sleep-related hypoventilation in children. In R. D. Chervin (Ed.). UpToDate.
https://www.uptodate.com/contents/congenital-central-hypoventilation-syndrome-and-other-causes-of-sleep-related-hypoventilation-in-children -
Paruthi, S. (2022, April 15). Evaluation of suspected obstructive sleep apnea in children. In R. D. Chervin. (Ed.). UpToDate.
https://www.uptodate.com/contents/evaluation-of-suspected-obstructive-sleep-apnea-in-children